Paediatric Research Services
Home /
To access the EPTRI services is easy!
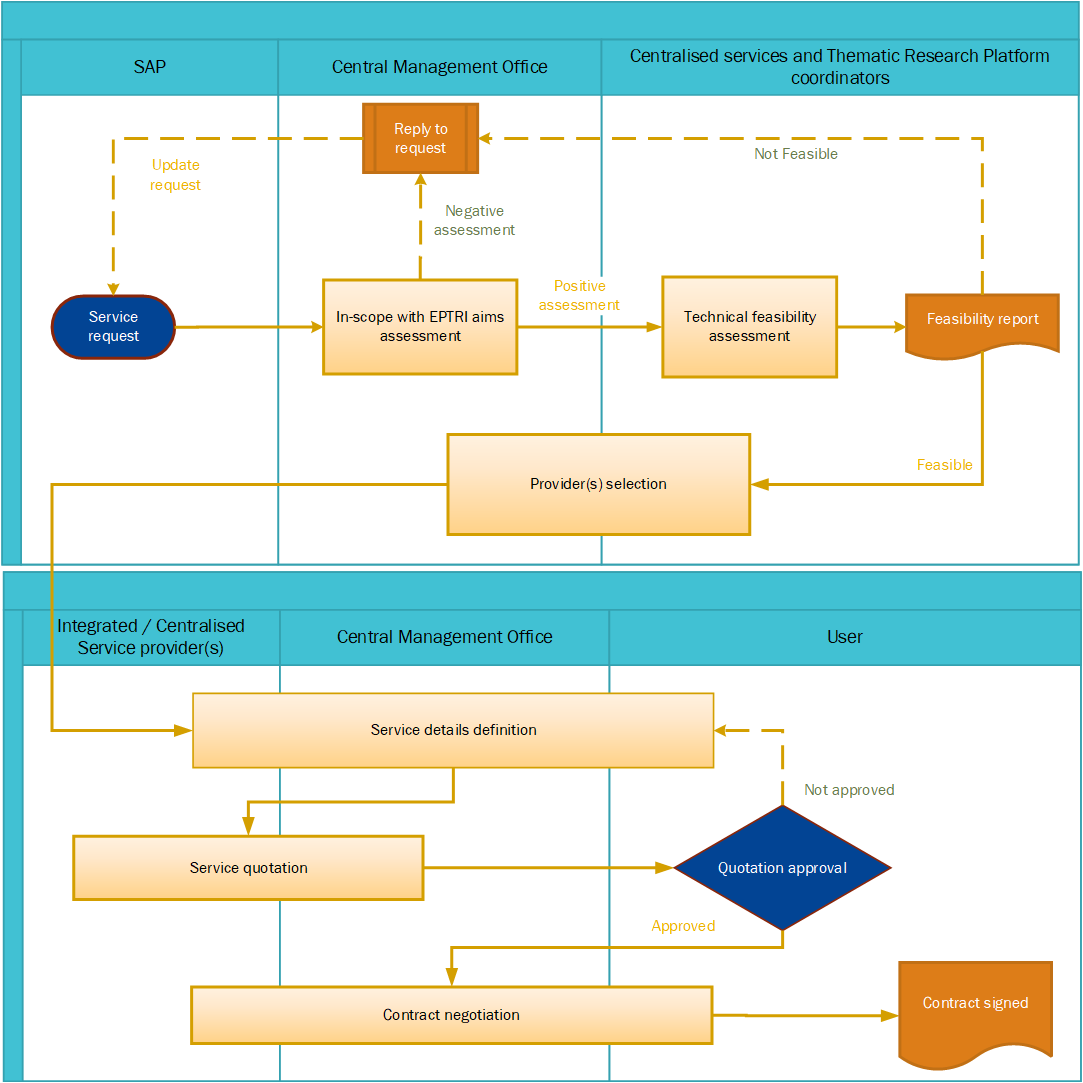
You can download the Service Request Form and the Confidentiality Disclosure Agreement (CDA) and fill them in all their parts to specify the request and its context and to protect the information you are sharing with EPTRI, respectively. Then send the form and the signed CDA to: [email protected].
Once received, your request form will be first evaluated by the CMO to assess if the proposal is in scope with EPTRI aims and then by the Centralized Services and Thematic Research Platform’s Coordinators to evaluate the technical feasibility of the service/s requested.
The procedure for provider’s selection and service assignment will be based on the provider’s expertise, technology capabilities, compliance with national and international standards, availability to provide the service(s) according to the User’s needs. For more details, the EPTRI Service Access Policy is available here.
If your service is not aligned to the EPTRI principles and procedures, we will inform you about the final decision.
The requested personal data will be only collected to allow the processing of the request. The information provided in the form will be available to EPTRI Coordinators for service provision purposes, such as the identification of the potential provider/s of the requested service/s that better match the requirements and needs of the user. For more details, the EPTRI Data Protection Policy is available here.
Who can access? The services are addressed to Pharmaceutical and Medical Device companies and SMEs, CROs, Healthcare organizations, Universities, Research Centers, Hospitals, Charities, Patients/Parents Organisations, etc. willing to find the right solution to their basic, pre-clinical and translational needs of research services!