OrphaDev4Kids will grant access to a complex ecosystem including tools, facilities, and supportive services (e.g. regulatory advice, assistance for prototype development, certification and marketing authorization, etc.) aimed to maximise the value-for-patients of the research and development of paediatric MDs projects.
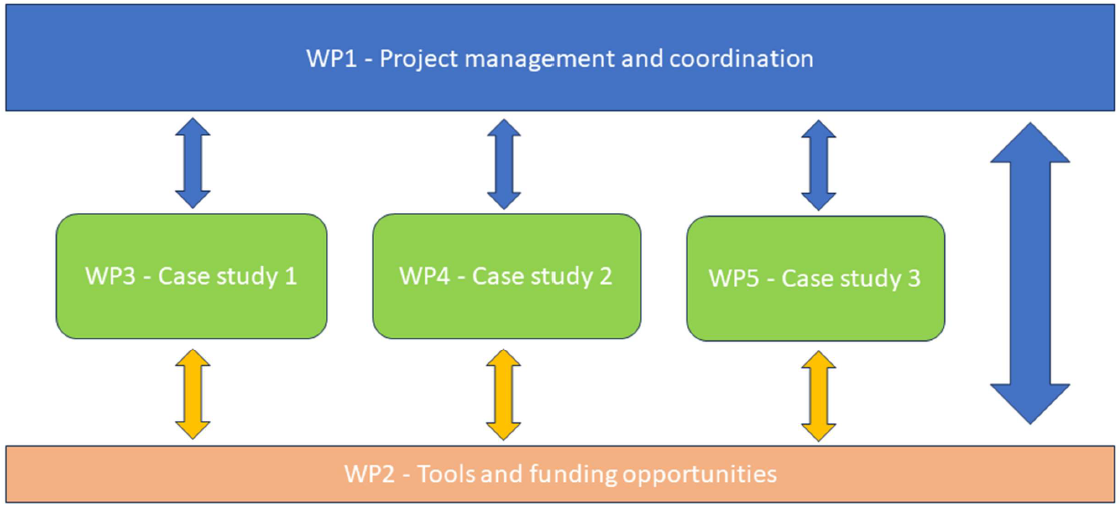
Project’s Work Plan
Open Call to support paediatric and orphan medical device development

The OrphaDev4Kids Project aims to grant access to an ecosystem including tools, facilities, and supportive services (e.g. funding advice, network building, technical advice, regulatory guidance) to advance medical device development for orphan and paediatric populations.
OrphaDev4kids invites academics and individual researchers, startups and industrial players, organizations from diverse settings, and patient-led developers, to request for supporting services both for new device development and for the adaptation of existing products for the orphan and paediatric populations.
Successful applicants will benefit from the support of dedicated experts to help achieve their project’s development goals.
To submit a request for supporting services for orphan and paediatric medical device development please fill in the Service Request Form, specifying the services you are interested to, sign it, upload the Confidentiality Disclosure Agreement and submit the request.
Please note that in the Service Request Form we are collecting all the services users might be interested in, but only funding advice, network building, technical advice and regulatory guidance will be provided by OrphaDev4Kids.
Partners
The OrphaDev4Kids consortium brings together a multidisciplinary group of nine partners from across Europe: